The Only Thing More Amazing Than
Polyurethane Is How You Use It
The Only Thing More Amazing Than Polyurethane Is How You Use It
We collaboratively design, precisely mold, and dependably deliver polyurethane components that improve your product’s performance.
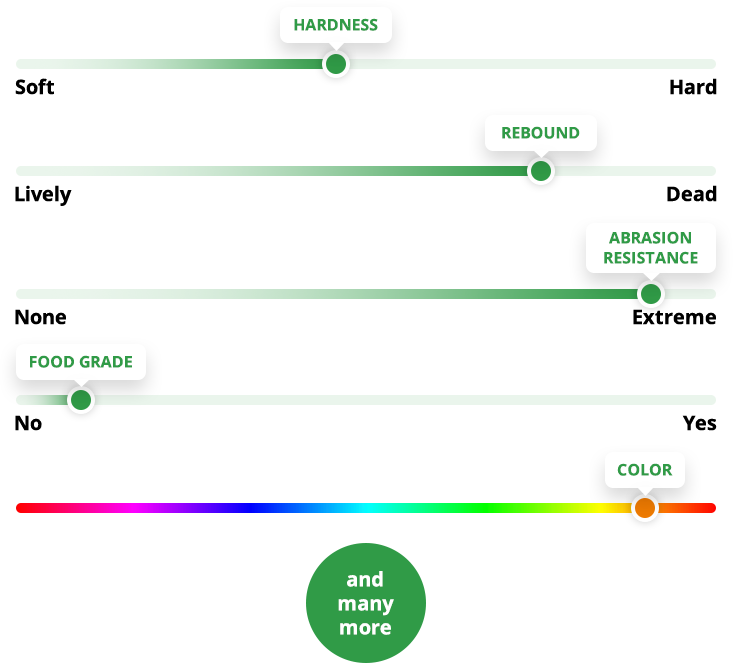
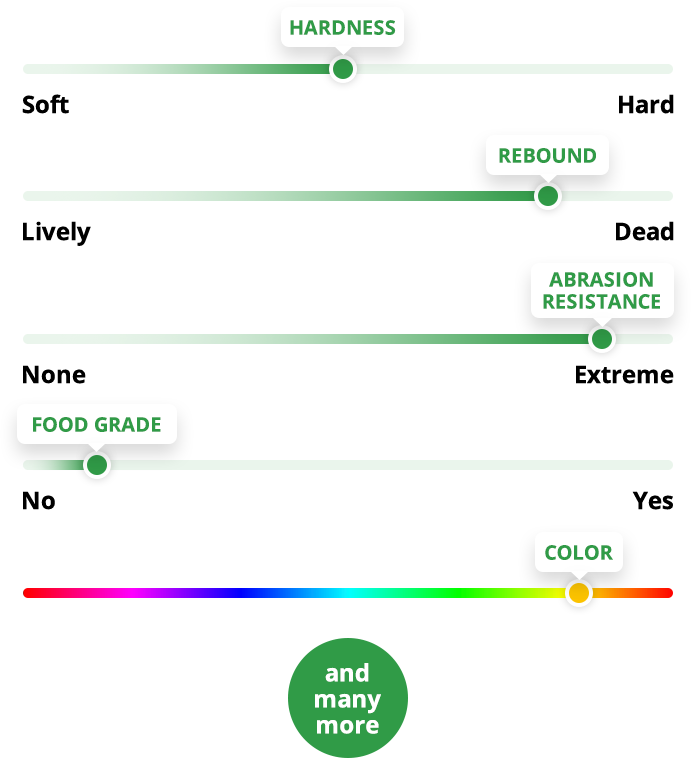
Countless applications because of three key advantages

Abrasion &
Wear Resistance
Polyurethanes have outstanding abrasion resistance, often outwearing corresponding parts made of metal, plastic, or rubber by a wide margin.

Dynamic Load Bearing and Impact Resistance
Polyurethanes have an excellent load-bearing capability and exhibit deflection and recovery that far exceed plastic or metal.

Complex Shapes
& Bonding
Creating complex shapes is no problem for polyurethane because we mold it in its liquid state. Polyurethane can be permanently attached — or bonded — to metals, plastics, and composites during the molding process.
Performance Properties Tailored To Your Application
We formulate the polyurethane to match your requirements. What can we customize for you?
You’ll Succeed With Us
We know polyurethane, how to mold it, and how to turn it into custom products. Which means that we can unlock the potential of polyurethane to solve your challenges.
Superior products dependably delivered. We deliver quality polyurethane products on time - in quantities from prototypes to millions.
Together we'll acheive success. As your polyurethane manufacturer, we'll help you lead your market.

Disrupting industries… It’s in our DNA
The beginning of our custom polyurethane molding services:
Starting with a Golf Ball
The first golf ball was an improvised pebble. From there, its construction advanced to using beech wood, then leather, then tree sap. Now the golf ball is a high-tech combination of core and cover.
Today’s best golf balls use an extremely durable polyurethane cover. Compared to other materials, polyurethane provides higher spin rates, better control, and a softer feel. It delivers performance that would have been unimaginable back in golf’s early days.
Golfers can thank Richard (Dick) Gallagher. Dick was the inventor of the polyurethane golf ball cover.


A Dream is Born
As a DuPont chemist, Dick developed and commercialized the earliest cast polyurethane materials. After the patent of the golf ball cover, he became more intrigued by the untapped potential of polyurethane to disrupt products. In 1965, Dick founded Gallagher Corporation to provide custom urethane molding services.
His mission: Mold polyurethane into game-changing products for OEMs across all industries.
Following Through on the Mission
Many new faces, and two more Gallagher generations, have joined the team. Today, we support hundreds of OEMs with our custom polyurethane molding services and products, across a variety of industries.
Our strategy remains the same — leverage the advantages of polyurethane to provide longer lasting and higher performing products that change industries.

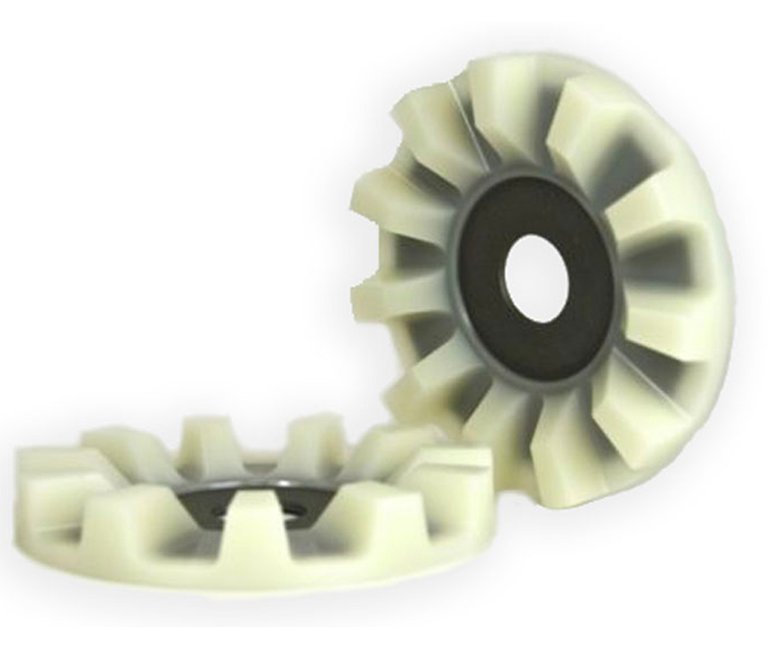
Case in Point: the John Deere Cotton Doffer
The doffer is one of two critical components that work together to pick cotton. First, barbed spindles rotate at high speed to remove the seed-cotton from the plant. Then, counter-rotating doffers pull the seed-cotton off of the spindles. It is a tough job subject to abrasive field conditions.
The problem John Deere faced was two-fold. First, the rubber doffers wore too quickly. Second, the black fragments that wore off the rubber doffers contaminated the white cotton harvest.
We worked with John Deere to understand the doffer application. Then we went to work on a custom polyurethane formulation to match their requirements. There were prototypes, field tests, and more prototypes. In the end, we significantly improved doffer wear life and eliminated the black rubber contamination. Our polyurethane doffer has been the industry standard for the past 30 years.
But it’s not about us. We’re happy to have made a contribution to Johnny Deere’s market leadership.